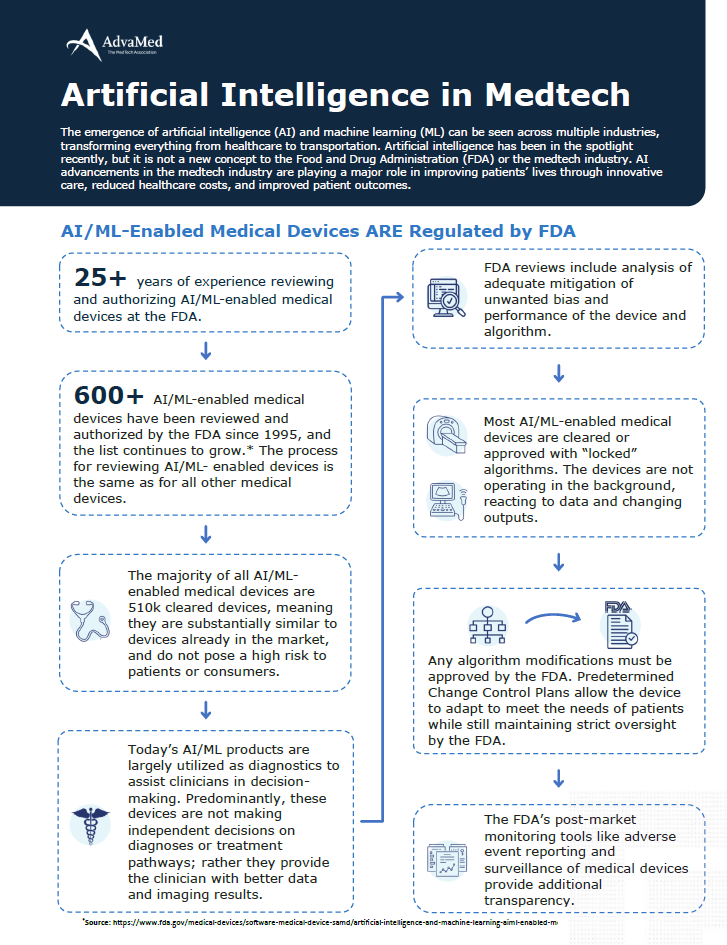
The emergence of artificial intelligence (AI) and machine learning (ML) can be seen across multiple industries, transforming everything from healthcare to transportation. Artificial intelligence has been in the spotlight recently, but it is not a new concept to the Food and Drug Administration (FDA) or the medtech industry. AI advancements in the medtech industry are playing a major role in improving patients’ lives through innovative care, reduced healthcare costs, and improved patient outcomes.
Related Reading
News / Artificial Intelligence (AI) / Coverage & Payment / Diagnostics / Digital Health / Government & Legislative Affairs / Medical Imaging / Regulatory Affairs
Medical Imaging Division Announces Rich Fabian as New Board Chair
January 16, 2026
WASHINGTON, D.C.—AdvaMed, the Medtech Association, today announced that Rich Fabian, president and CEO of FUJIFILM Sonosite, will serve as the chair of the Medical Imaging division’s board of directors. He will succeed David Pacitti, CEO of Avanos Medical and former president and head of the Americas at Siemens Healthineers, the inaugural division board chair.
News / Artificial Intelligence (AI) / Coverage & Payment / Diagnostics / Digital Health / Government & Legislative Affairs / Orthopedic / Regulatory Affairs
Robert Cohen of Stryker Named Chair of AdvaMed’s Digital Health Tech Division Board of Directors
January 5, 2026
WASHINGTON, D.C.—AdvaMed, the Medtech Association, today announced that Robert Cohen, vice president, innovation and technology, orthopaedic group at Stryker, will be the next chair of the AdvaMed Digital Health Tech Board of Directors. He succeeds Dr. Taha Kass-Hout, global chief science and technology officer at GE HealthCare, who served as the inaugural chair of the board overseeing the then-newly created division.
News / Artificial Intelligence (AI) / Coverage & Payment / Diagnostics / Digital Health / Government & Legislative Affairs / Medical Imaging / Orthopedic / Regulatory Affairs / Small Business
Mick Farrell, Chairman and CEO of Resmed, Named Chair of AdvaMed Board of Directors
December 11, 2025
WASHINGTON, D.C.—AdvaMed, the Medtech Association, today announced Michael “Mick” Farrell, chairman and CEO of Resmed Inc. (NYSE: RMD, ASX: RMD), the leading health technology company focused on sleep, breathing, and care delivered in the home, will be the next chair of the AdvaMed Board of Directors. Farrell will serve a two-year term beginning in January 2026.
Event / Regulatory Affairs
Medical Device Submissions Series: Investigational Device Exemption (IDE) Workshop
8:30 am – 5:15 pm ET
February 25, 2026
Our in-depth device submissions workshops will provide real-world case studies, tips and best practices directly from FDA and industry experts.
Event / Regulatory Affairs
Medical Device Submissions Series: Premarket Approval (PMA) Workshop
8:30 am – 4:30 pm ET
February 26-27, 2026
Learn key PMA Submissions strategies to build a strong submissions, align with FDA expectations, and reduce delays.
Event / Regulatory Affairs
Medical Device Submissions Series: 510(k) & De Novo Workshop
8:30 am – 4:15 pm ET
February 23-24, 2026
Join industry leaders for the in-depth 510(k) & De Novo Workshop for real-world case studies, submissions tips and best practices.
News / Government & Legislative Affairs / Health Access / Regulatory Affairs
AdvaMed Urges CMS to Protect Patient Access to Critical Medical Equipment
November 30, 2025
WASHINGTON — AdvaMed, the medtech association, the world’s largest trade association representing medtech innovators, today released the following statement on the U.S. Centers for Medicare & Medicaid Services’ (CMS) release of its 2026 Home Health Prospective Payment System final rule:
Event / Regulatory Affairs
QMSR Transition: Legal and Audit Perspectives
December 9, 2025
11:00 AM – 12:00 PM
Join Hogan Lovells experts to learn how the FDA is set to reshape the medical device regulatory landscape with the introduction of the QMSR.