
Nominate a Medtech Visionary for AdvaMed®’s 2025 Lifetime Achievement Award
Nominate a medtech pioneer for AdvaMed®’s 2025 Lifetime Achievement Award to celebrate their contributions to the health care industry!
AdvaMed®’s Medtech POV blog features all the latest developments, perspectives, and resources by and for the medical technology community.

Nominate a medtech pioneer for AdvaMed®’s 2025 Lifetime Achievement Award to celebrate their contributions to the health care industry!

We took time to reflect on another extraordinary year in the medtech industry and the tremendous strides we’ve made together. Our 2024 AdvaMed® Annual Report highlights our key policy wins,…
AdvaMed® filed an amicus brief in the case of Smith v. Terumo BCT, Inc. The case concerns whether the court should create a cause of action for asymptomatic plaintiffs, permitting…
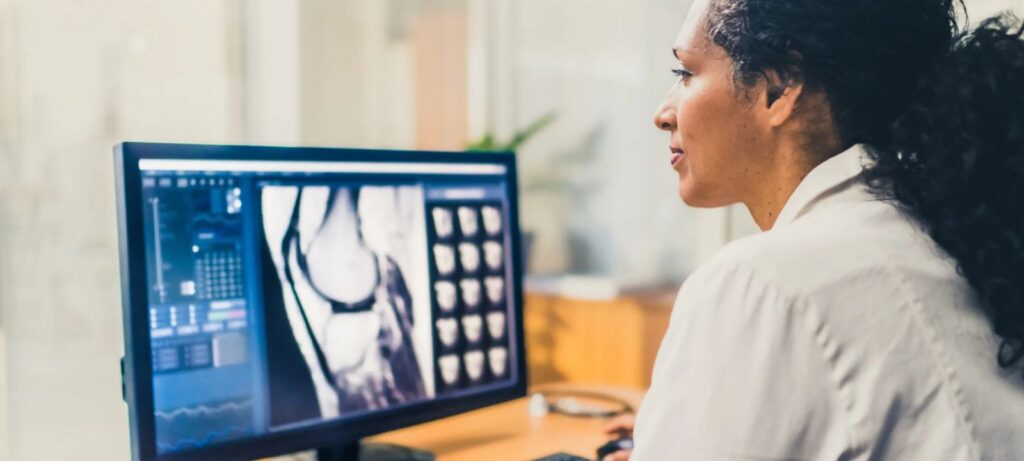
Recap AdvaMed®’s Medical Imaging Divisions achievements for members and the industry during its inaugural year with the medtech association.
Brought to you by AdvaMed®, Medtech POV with Scott Whitaker is a podcast that covers the intersection of medtech and policy from every perspective.
This case involves a patent infringement dispute between EcoFactor and Google, but the Court’s decision will reach every aspect of district court patent litigation in cases involving parties in every…

Maria Artunduaga’s grandmother never smoked. However, like many members of her generation, she was exposed to second-hand cigarette smoke much of her life. When she developed chronic obstructive pulmonary disease…
The issue at hand is patent infringement and an overly broad interpretation of the Hatch-Waxman Act’s safe harbor. AdvaMed® pointed out that this would harm innovation. The decision has profound…

Our health care system moves at the speed of medtech innovation – and from my vantage point, that momentum is meteoric. We are living through a profound evolution in health…
If you have media inquiries, please contact:
Cody Uhing, Director, Communications & Media Relations, 202-316-1423

The outcome of the 2024 presidential election stands to have a lasting impact on American health care. Questions surrounding artificial intelligence (AI) and its appropriate role in our society and…
Join over 500 medical technology companies as a member of one of the most effective trade associations in Washington, D.C.