MDUFA V Enacted, Ushering in Next Era of Medtech for Patients
- Scott Whitaker President & Chief Executive Officer

Every five years, Congress must pass, and the President must sign into law, an agreement between the medical technology industry and the FDA on the fees medtech companies pay toward giving the FDA the resources it needs to review device submissions in a timely, efficient, and cost-effective way. Following quick work by the Senate, the House of Representatives overwhelmingly approved this year’s agreement. President Biden then signed the legislation into law.
This is the fifth Medical Device User Fee Amendment (MDUFA) agreement, known in the Washington, D.C., world as “MDUFA V (Five),” to go into effect since the user-fee system was first implemented in 2002.
The agreement builds on the successes of prior agreements for several historic steps that will usher in a new golden age of innovation for the patients medtech companies treat and serve.
“This agreement ensures that current and future medtech leaders have the best platform to launch their lifesaving technologies.”
––Scott Whitaker, President and CEO, AdvaMed®
Key Improvements in MDUFA V
Overall, the agreement reflects the FDA’s ever-increasing workload, as the COVID-19 pandemic brought into focus, and as medtech innovators continuously present new products for FDA review.
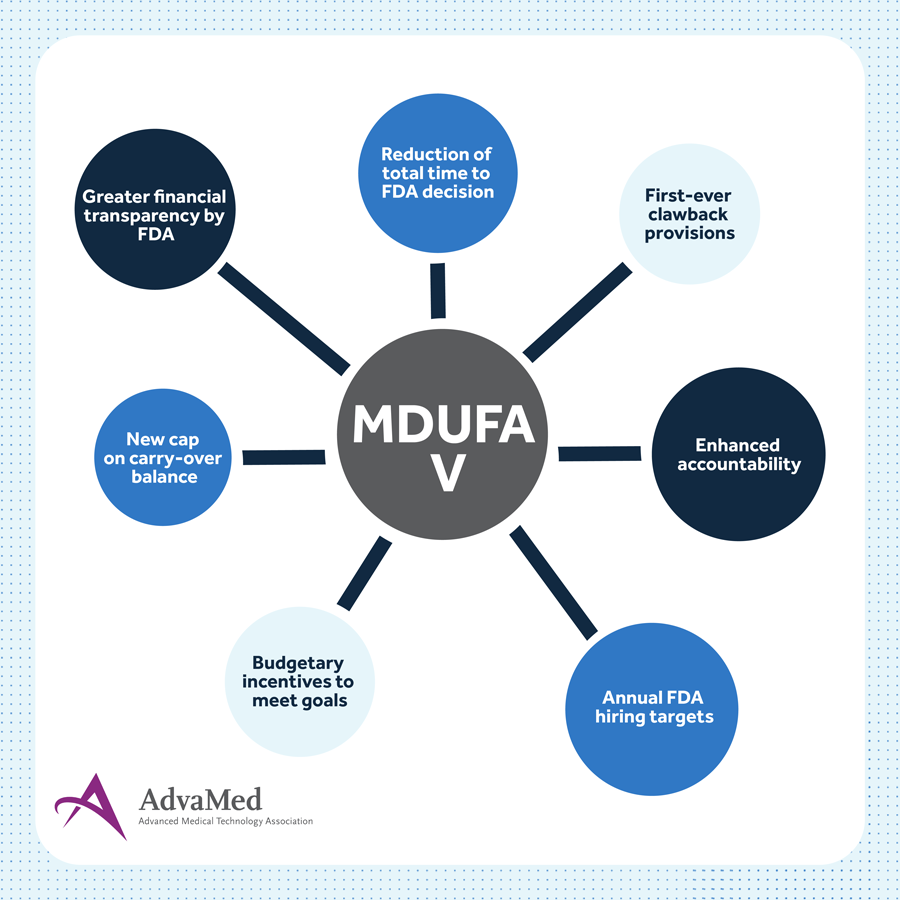
MDUFA V increases the FDA’s base funding level to help meet the increased device review submissions and gives the FDA the opportunity for additional incentive funding if the agency meets key performance targets defined by this new law. New steps include:
- FDA will be required to meet annual hiring targets. Not meeting these targets will result in unused funds being returned to the industry in the form of lower facility registration fees.
- New timelines to reduce the total time to FDA decision by 20 days through the 510(k) and premarket approval pathways, speeding up patient access to new technologies if approved.
- Budgetary incentives for the FDA to meet the agreement’s goals.
- A new cap on carry-over balances. Any over-collection will result in reduced facility registration fees in the next fiscal year.
The next five years look to be a revolutionary time in health care, and medtech companies are at the forefront of innovation-driven changes that will improve patient care. This agreement ensures that current and future medtech leaders have the best platform to launch their lifesaving technologies. AdvaMed® is grateful for the timely work of the legislative and executive branches in bringing MDUFA V to fruition for patients.
Scott Whitaker is AdvaMed® president and CEO.
Hear Patient Stories
The Story of Medtech empowers patients to share their experiences with medical technology in an effort to educate, inspire, and create community.