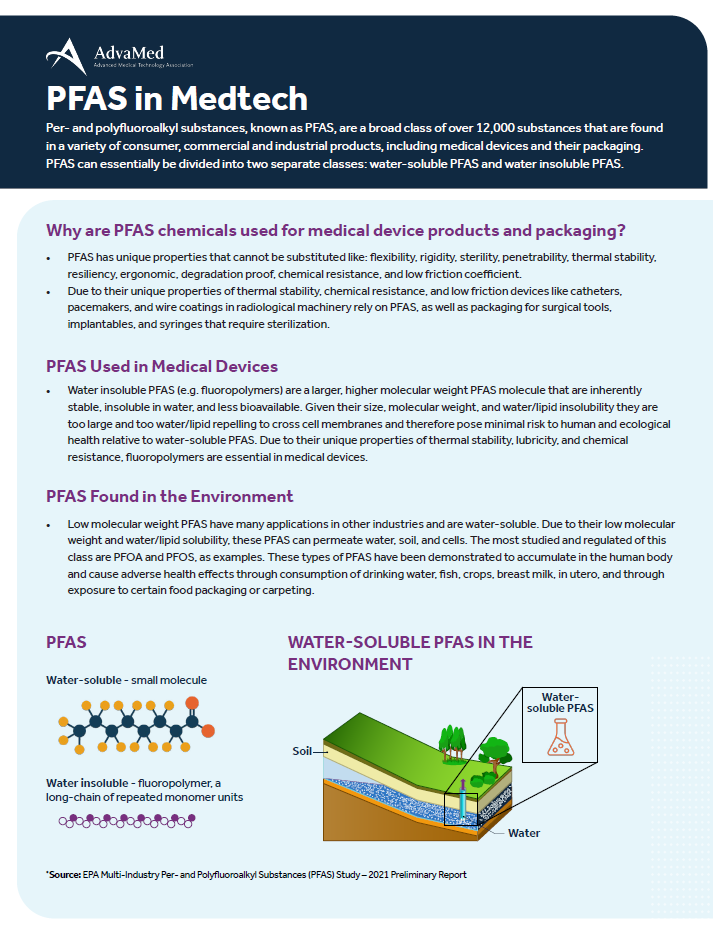
Per- and polyfluoroalkyl substances, known as PFAS, are a broad class of over 12,000 substances that are found in a variety of consumer, commercial and industrial products, including medical devices and their packaging. PFAS can essentially be divided into two separate classes: water soluble PFAS and water insoluble PFAS.
Learn more about PFAS in medical technology in our briefing document found on this page and in the video below.
Related Reading
Blog / Global & Trade / Government & Legislative Affairs / Regulatory Affairs / Tariffs
Medtech is Essential: Industry Supports Call for Tariff Flexibility
May 29, 2025
AdvaMed President and CEO Scott Whitaker testified before the U.S. Senate Finance Committee, bringing attention to the pressing issue of how tariffs could impact the medtech industry and, most importantly, the patients who depend on it.
Blog / Government & Legislative Affairs / Regulatory Affairs
Orthogonal, Kwame Ulmer on the Value of AdvaMed Membership
May 20, 2025
At AdvaMed, we’re proud to be recognized for our work on behalf of the medtech community – and we’re especially grateful when that recognition comes from respected voices like Kwame Ulmer of MedTech Impact Partners and our colleagues at Orthogonal.
News / Diagnostics / Digital Health / Government & Legislative Affairs / Regulatory Affairs
AdvaMed Releases “AI Policy Roadmap” to Guide Congress and Federal Agencies
April 22, 2025
Washington, D.C.—AdvaMed, the Medtech Association, today released its “AI Policy Roadmap,” a detailed policy outline for Congress and the federal agencies with jurisdiction over medtech to consider in promoting the next era of artificial intelligence-enabled medical and digital health technologies to provide patients and clinicians transformative new tools to aid in diagnosis and treatment.
News / Coverage & Payment / Government & Legislative Affairs / Regulatory Affairs
AdvaMed Congratulates Dr. Oz on his Confirmation as CMS Administrator
April 3, 2025
Washington, D.C.– AdvaMed®, the Medtech Association, today released the following statement from President and CEO Scott Whitaker on the U.S. Senate’s confirmation of Dr. Mehmet Oz to serve as Administrator of the U.S. Centers for Medicare and Medicaid Services.
Resource / Regulatory Affairs
Medical Device Submissions Guidebook: 510(k) & De Novo
April 2, 2025
This in-depth 510(k) & De Novo Guidebook will provide real-world case studies, tips and best practices directly from FDA and industry experts.
Blog / Coverage & Payment / Diagnostics / Global & Trade / Government & Legislative Affairs / Regulatory Affairs / Small Business
AdvaMed®’s Medical Innovation Agenda for the 119th Congress
March 28, 2025
AdvaMed®’s medtech priorities for the 119th Congress highlight opportunities to address the most important issues facing patients and the medical technology industry today.
Resource / Compliance / Global & Trade / Regulatory Affairs
State of the Nation 2025: Global Medical Device Recall Index Report
March 27, 2025
Download Sedgwick’s Global Medical Device Recall Report for key insights on compliance, regulations, and global recall trends. Members only.
Event / Compliance / Coverage & Payment / Regulatory Affairs
Richardson Waiver Repeal: What it Means for Medtech
Now On Demand
Sidley Austin, joined by HPA, will lead a program examining the impact of HHS repealing the so-called “Richardson Waiver”, including its history, how it could impact the activities of the U.S. Department of Health and Humans Services (HHS), what it all means for medtech and its patients.