Fact Sheet: EtO Sterilization Prevents Infection
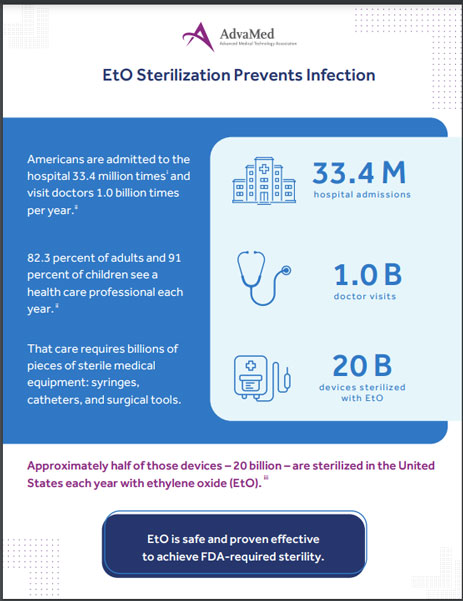
Ethylene oxide sterilizes 20 billion medical devices in the United States each year. Sterilized equipment is critical to preventing serious, even life-threatening, infections in patients at the doctor and in the hospital every day.
Related Reading
Event / Emerging Policy Response Resources / Global & Trade / Government & Legislative Affairs / Supply Chain / Tariffs
The Kennedy v Braidwood Ruling: What’s Next for MedTech
July 17, 2025
2:00 PM – 3:30 PM
Join AdvaMed and Reed Smith experts to explore how the Supreme Court’s ACA ruling in Braidwood could shape preventive care and MedTech policy.
News / Government & Legislative Affairs
AdvaMed Statement on the Tax Bill
July 3, 2025
WASHINGTON – Today, AdvaMed, the Medtech Association, released the following statement from President and CEO Scott Whitaker on the impact the “One Big Beautiful Bill Act” will have on medtech innovation.
Blog / Diabetes / Diagnostics / Digital Health / Government & Legislative Affairs / Medical Imaging / Orthopedic / Radiation Therapy
Medtech Goes to Capitol Hill
June 26, 2025
AdvaMed’s annual Medtech Showcase and Reception on Capitol Hill seeks to inform a core audience: the members of the Congress and their staff who enact the policies that help our member companies reach patients with their transformative medtech.
Blog / Global & Trade / Government & Legislative Affairs / Regulatory Affairs / Tariffs
Medtech is Essential: Industry Supports Call for Tariff Flexibility
May 29, 2025
AdvaMed President and CEO Scott Whitaker testified before the U.S. Senate Finance Committee, bringing attention to the pressing issue of how tariffs could impact the medtech industry and, most importantly, the patients who depend on it.
Event / Emerging Policy Response Resources / Global & Trade / Government & Legislative Affairs / Supply Chain / Tariffs
URGENT: Court Ruling on Tariffs — Implications for Medtech
Now On Demand!
Emergency Briefing on Court Ruling on Tariffs and Its Impact on Medtech. Now On Demand!
News / Business Development / Government & Legislative Affairs / Small Business
AdvaMed Welcomes Introduction of American Innovation and Jobs Act
May 22, 2025
WASHINGTON—AdvaMed, the Medtech Association, welcomed introduction of the bipartisan Access to Prescription Digital Therapeutics Act of 2025 (S. 1702/H.R. 3288) by U.S. Sens. Shelley Moore Capito (R-W.Va.) and Jeanne Shaheen (D-N.H.) and U.S. Reps. Kevin Hern (R-Okla.) and Mike Thompson (D-Calif.). The bill provides for Medicare and Medicaid coverage of evidence-based software applications used to prevent, manage, or treat medical conditions.
News / Coverage & Payment / Digital Health / Government & Legislative Affairs
AdvaMed Welcomes Introduction of Access to Prescription Digital Therapeutics Act
May 22, 2025
WASHINGTON—AdvaMed, the Medtech Association, welcomed introduction of the bipartisan Access to Prescription Digital Therapeutics Act of 2025 (S. 1702/H.R. 3288) by U.S. Sens. Shelley Moore Capito (R-W.Va.) and Jeanne Shaheen (D-N.H.) and U.S. Reps. Kevin Hern (R-Okla.) and Mike Thompson (D-Calif.). The bill provides for Medicare and Medicaid coverage of evidence-based software applications used to prevent, manage, or treat medical conditions.
News / Coverage & Payment / Government & Legislative Affairs
AdvaMed Welcomes the Re-Introduction of the Ensuring Patient Access to Critical Breakthrough Products Act
May 22, 2025
Washington, D.C.–AdvaMed President and CEO Scott Whitaker released the following statement upon re-introduction of bipartisan legislation aimed at accelerating Medicare coverage for breakthrough medtech and diagnostics. The Ensuring Patient Access to Critical Breakthrough Products Act, introduced by Senators Todd Young (R-Ind.) and Alex Padilla (D-Calif.), allows Medicare beneficiaries with life-threatening or debilitating diseases or conditions access to groundbreaking medical innovations, designated as breakthrough devices to treat unmet needs and already authorized by the FDA.