Fact Sheet: EtO Sterilization Prevents Infection
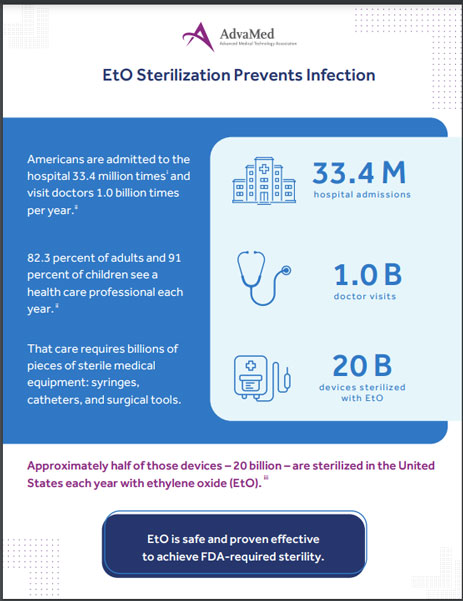
Ethylene oxide sterilizes 20 billion medical devices in the United States each year. Sterilized equipment is critical to preventing serious, even life-threatening, infections in patients at the doctor and in the hospital every day.
Related Reading
Blog / Diagnostics / Digital Health / Government & Legislative Affairs / Health Access
Heart Month: Why Sleep Apnea Belongs at the Center of Cardiovascular Care
February 13, 2026
February is American Heart Month—a time to spotlight cardiovascular disease and highlight innovations that can improve patient outcomes. For the medtech community, it’s an opportunity to address persistent gaps in care through technology, data, collaboration, and policy advancement. Despite progress in treating heart conditions, sleep disorders—especially sleep apnea—remain a significant and underrecognized contributor to poor cardiovascular outcomes.1 Tackling this issue requires clinical awareness and supportive policies for timely diagnosis, coverage, and care beyond traditional settings.
News / Amicus Briefs / Ethylene Oxide / Legal
AdvaMed Urges Georgia Court of Appeals to Enforce Sound Science in Ethylene Oxide Litigation
February 13, 2026
WASHINGTON—AdvaMed, the Medtech Association, the world’s largest medtech trade association, representing over 650 medical technology innovators, has filed an amicus curiae brief in the Walker v. Becton, Dickinson and Company and C. R. Bard, Inc. case now before the Georgia Court of Appeals, urging the Court to reverse a trial court ruling that allowed unreliable expert testimony on ethylene oxide (EtO) exposure.
News / Coverage & Payment / Diagnostics / Regulatory Affairs
AdvaMed Announces Melissa Torres as New Executive Vice President of Technology and Regulatory Affairs
February 10, 2026
WASHINGTON—AdvaMed, the Medtech Association, today announced Melissa Torres is the new Executive Vice President of Technology and Regulatory Affairs. Torres succeeds Janet Trunzo, who retired after 30 distinguished, productive years with AdvaMed.
News / Diagnostics / Digital Health / Medical Imaging / Regulatory Affairs
AdvaMed Receives RAPS Accreditation for Medical Device Submissions Workshop Series (February 2026 in Washington, D.C.)
February 4, 2026
WASHINGTON, D.C.—AdvaMed, the world’s largest trade association representing medical technology innovators, announced that its Medical Device Submissions Workshop Series (February 23-27 in Washington, D.C.) has been officially approved for RAC recertification credits by the Regulatory Affairs Professionals Society (RAPS).
Blog / Diagnostics / Digital Health / Regulatory Affairs / Standards
Honoring Janet E. Trunzo: A Legacy That Shaped Modern Medtech Regulation
January 29, 2026
After 30 years at AdvaMed, Janet E. Trunzo, Senior Advisor to the President and Senior Executive Vice President of Technology and Regulatory Affairs, will retire on January 31, 2026. Her tenure has fundamentally shaped how medical devices are regulated in the United States and globally, establishing frameworks and standards that continue to govern the field today.
Blog / Regulatory Affairs
Strengthen Your IDE Strategy at AdvaMed’s IDE Submissions Workshop
January 27, 2026
Gain in-depth IDE submission training, meaningful face time with FDA and regulatory leaders, and RAPS accreditation credits at AdvaMed’s IDE Submissions Workshop.
Blog / Artificial Intelligence (AI) / Coverage & Payment / Diagnostics / Digital Health / Government & Legislative Affairs / Health Access / Medical Imaging
Why the Health Tech Investment Act Matters: Peter Weems Breaks Down What This Means for Patients
January 26, 2026
In a new video developed in partnership with Patients Rising, AdvaMed’s Peter Weems, Vice President of Imaging Government Affairs and Policy Strategy, explains why the Health Tech Investment Act is critical to expanding patient access to FDA-authorized AI-enabled medical devices.
News / Coverage & Payment / Diagnostics / Regulatory Affairs
AdvaMed Names Brian J. Blaser, QuidelOrtho President and CEO, Chair of AdvaMedDx Board of Directors
January 21, 2026
WASHINGTON, D.C.—AdvaMed, the Medtech Association, today announced that Brian Blaser, president and CEO of QuidelOrtho, will serve as the next chair of the AdvaMedDx Division Board of Directors, succeeding Thierry Bernard, CEO of QIAGEN. AdvaMedDx represents more than 70 manufacturers of the innovative in vitro diagnostic (IVD) tests critical to patient care.