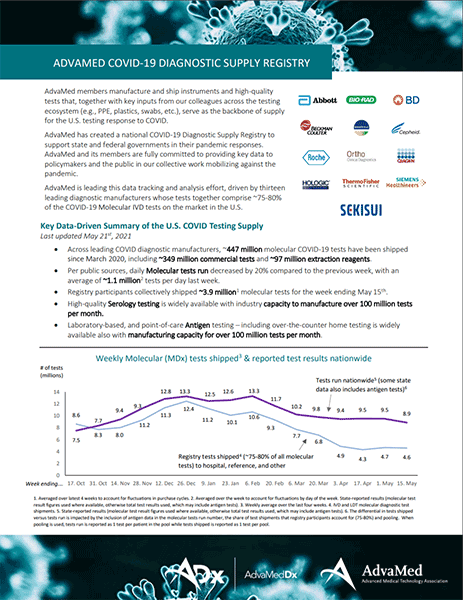
AdvaMed members manufacture and ship instruments and high-quality tests that, together with key inputs from our colleagues across the testing ecosystem (e.g., PPE, plastics, swabs, etc.), serve as the backbone of supply for the U.S. testing response to COVID. AdvaMed has created a national COVID-19 Diagnostic Supply Registry to support state and federal governments in their pandemic responses. AdvaMed and its members are fully committed to providing key data to policymakers and the public in our collective work mobilizing against the pandemic.