Getting to the Heart of Cardiac Innovation: Dr. Ajit Yoganathan’s Story
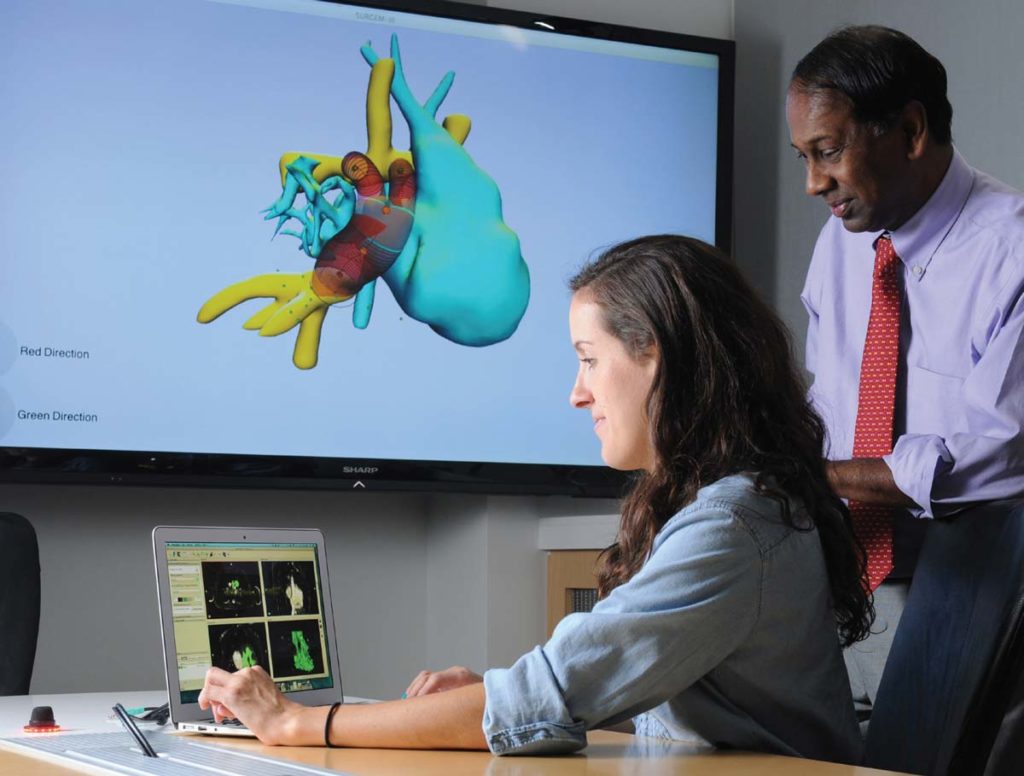
Ph.D. student Maria Restrepo works with a computer simulation program that enables pediatric heart surgeons to run virtual tests of surgical options for repairing severe heart defects. Photo credit: Gary Meek
The medical technology community is made up of scientists, manufacturers, researchers, and engineers. Dr. Ajit Yoganathan is a unique combination of them all. Even so, Dr. Yoganathan – or Dr. Y to his colleagues and friends – would describe himself using an entirely different, but apt moniker: A conductor.
“This work that has happened wouldn’t have happened without the clinicians I collaborated with, without the students and trainees in the labs,” he said. “They’re the ones who got the work done and got it out there. I orchestrated, I gathered the resources and tried to help people, but the actual music was created by the people in the labs.”
Of course, that “music” is metaphor, but it’s still a masterpiece: Dr. Y and his teams have spent more than 40 years inventing and improving the science of prosthetic heart valve design to help people with heart disease.
A Lucky – But Lasting – Start in Medical Research

Dr. Y didn’t intend on a career in health care – it just happened.
“In 1973, I came to Cal Tech to do my PHD in chemical engineering, with the idea of doing something in the area of air pollution, because that was a hot topic at the time and an area where there was a lot of research going on,” he said.
But during his initial few months on campus, a very different faculty research project piqued his interest.
“These faculty were looking at blood flow, related to the sound that is created in the blood pressure cuff when the doctor takes your blood pressure,” Dr. Y explained. “It kind of interested me, because I never thought of engineering having an impact that way on medicine. I came from a medical background – both my parents were doctors – so I knew a lot but wasn’t imagining medicine as a career for me. Then, I get this opportunity to see how maybe engineering like I was doing could intersect with medicine.”
Just a few months after joining the project, Dr. Y arrived at that intersection, and saw just how impactful it could be. The research team was approached by a cardiologist at one of the largest heart valve clinics in the country, and he needed their help.
“Dr. Earl Harrison was running the valve clinic at University of Southern California, LA County, and was interested in diagnosing valve thrombosis,” Dr. Y shared.
Prosthetic valve thrombosis is a serious complication of a valve replacement, where a blood clot attaches to the prosthetic heart valve, obstructing blood flow and interfering with the function of the valve itself. Unfortunately, at the time, it was a difficult complication to diagnose.
“The mechanical valve wouldn’t function properly if a patient had developed thrombosis. And we found we were able to differentiate between a functioning and a non-functioning valve using our sound analysis. A healthy valve makes a clear clicking sound when it opens and closes. When a valve is obstructed by a blood clot – or other biological material – that clicking sound is muffled. So, Dr. Harrison asked us: Can you use some of that analysis you’re doing to help us better diagnose valve thrombosis?”
The team got to work, and their work attracted the attention of other clinicians interested in their own partnerships.
“At the end of day, that’s what our research was about,” said Dr. Y. “Answering a question for a clinician who needed to make a diagnosis. Taking fundamental engineering, and fundamental physics, and applying it to a real-life problem. Can you provide a solution to the problem or can you provide answers to the question that would impact how a clinician helps the patient? Or come up with solutions that directly impact the patient? That’s translational research.”
From Medical Practitioners to the Medical Marketplace
Dr. Y’s translational research certainly translated – to new understandings that rippled far beyond academia. Dr. Y and his clinician partners were painting an ever-clearer picture of how the heart pumps blood – as well as how to correct problems with the blood-pumping process.
In the course of their work, one thing became clear: The medical technology manufacturers – the companies and innovators making prosthetic valves – needed to be a part of their progress.
“At that time, anyone who made a heart valve resided about 40 miles from Pasadena in Orange County,” Dr. Y said. “Orange County was full of orange groves and small, start-up companies. I started visiting them, and most of them were open to discission. I started developing relationships with them.”
Together, Dr. Y, his team, and their new industry partners began to improve prosthetic heart valves for patients across the country.
“At the time, someone could come up with a valve in their garage, stick it in a human, take it out, and try another one,” he said. “We wanted to take out a lot of that guesswork.”
Taking out the guesswork was still Dr. Y’s objective when he founded his own laboratory at Georgia Tech a few years later. But working with manufacturers wasn’t the only way to achieve it. It was time for Dr. Y to extend his collaboration once more, this time with government regulators.

New Standards for Success
“In 1976, Congress passed the Devices Act, establishing a regulatory environment for medical devices,” said Dr. Y. “Whether it was a class 1 device – like a bedpan – or a class 3 device – like a lifesaving heart valve, it would need to be regulated.”
The premise was simple enough, but putting it into action was a completely different story.
“In 1978, I ran into a couple of FDA engineers at a conference, and we got to talking,” said Dr. Y. “They were telling me: ‘We don’t have the mechanisms of understanding how to test these devices. We don’t know what’s appropriate.’ So, we sat down and started very informally putting together what would be the first set of guidelines FDA developed for heart valve testing on the bench.”
Not only did Dr. Y help shape those guidelines – as well as the new iterations of the International Standard on Cardiac Valve Prostheses (ISO 5840 Parts 1, 2 & 3) as recently as January 2021 – he helped ensure medical device manufacturers adapted to comply with these guidelines and standards.
“I was able to work very uniquely with both industry and FDA, especially when that relationship was fragile,” said Dr. Y. “I would talk to the companies, then talk to the FDA, and try to facilitate their interactions and policies to reflect the realities.”
Even as specific policies changed, the heart of the standards never wavered.
“Safety and efficacy are the cornerstones,” said Dr. Y. “From when I first joined the standard-making efforts to the latest evolution in January 2021, safety and efficacy have been the cornerstones.”
Taking Innovation from Bench to Bedside
With a new regulatory framework in place – and with heart valve innovation happening at breakneck speed – Dr. Y’s laboratory became busier than ever. Their work continues to pay dividends.
Since 1975, every prosthetic heart valve implanted in the U.S. – more than a dozen valve designs – has been studied and evaluated in Dr. Y’s lab. His fingerprints are on the products from manufacturers like St. Jude Medical, Edwards Lifesciences, Medtronic, and Boston Scientific, as well as small and start-up biomedical companies.
Additionally, Dr. Y holds 19 U.S. patents, with another 7 patent applications under review. In October 2009, he licensed one of his patents to Edwards Lifesciences – who is subsequently developing a medical device to target heart valve repair. In November 2009, he co-founded APICA Cardiovascular, using co-founded patents to create and launch its first product: a “bloodless” ventricular access and closure device, which received CE mark approval in 2013. Another one of his inventions, a children’s continuous renal replacement therapy device, is in initial animal studies, previously funded by a NIH challenge grant and a FDA pediatric device center grant.
All of these innovations have served as building blocks for a brighter future for people with heart disease. Dr. Y imagines that by the end of this decade, we’ll start to see this foundation’s complete impact.
“It’s really exciting right now,” he said. “We have transcatheter devices, that are giving people who would traditionally have needed open heart surgery another option, a minimally-invasive option. At first, we were using these transcatheter devices only on extremely high-risk, inoperable, older patients, but now, as recently as last year, we’re using them on low-risk, younger patients. The innovation pipeline there is good.”
…and From Bench to Bassinet
till, Dr. Y can admit that the innovation pipeline isn’t perfect, especially when it comes to innovations for children.
“In the heart valve area, new innovations have been developed for years since the mid-1950s, but they’ve been addressing heart disease problems for adults, not children,” Dr. Y said. “There is a small but significant number of children who are born with abnormalities in their heart, and some of those can effect their heart valve. But that patient population is so small, it presents a big challenge to come up with devices that could be studied in a clinical environment. In other words, it’s hard to carry out a proper clinical trial according to the rules.”
So, Dr. Y explains, clinicians often have to make do what the products on the market for adults.
“Surgeons are free to do surgical manipulations,” he said. “They could adapt adult-sized products to fit their child patients. But children are not small adults. So, starting in late 2008, early 2009, we worked with FDA to get them to allow manufacturers to downsize approved adult-sized valves to a size appropriate for children with less required testing. Finally, by 2014 or 2015, St. Jude Medical, now Abbott Labs, delivered the first pediatric-sized valve.”
Dr. Y also delivered his own innovation into the mix: A surgical planning software for difficult cardiac surgeries in babies. Dr. Y’s software is able to predict how a specific surgery will affect the baby’s cardiac blood flow, and thus, predict how a specific surgery will correct or exacerbate the baby’s condition.
“It has been well utilized,” said Dr. Y. “I feel extremely proud of it. We have helped save kids’ lives.”
Lengthening a Legacy
It’s difficult to estimate exactly how many lives Dr. Y has saved and improved – but it’s safe to say, the number is quite high. And that number accounts for not just the patients he’s served, but also the students, trainees, and mentees he’s taught.
“I’m very proud of the people who have come out of my lab – PhD students, master’s students, post-doctoral trainees, even undergraduates that have worked in my lab,” said Dr. Y. “A number of them have gone onto industry, others have gone into academia to carry on this tradition of translational research.”
But to Dr. Y, it’s not about the job itself: it’s always about that impact.
“Their impact is what is important,” he said. “We put millions of dollars into R&D, but when one does a true accounting, what matters is how much is getting out to patients.”
Hear Patient Stories
The Story of Medtech empowers patients to share their experiences with medical technology in an effort to educate, inspire, and create community.